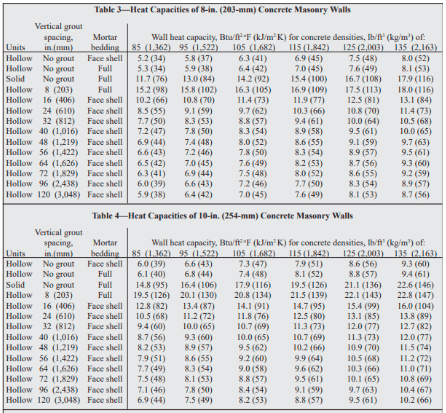
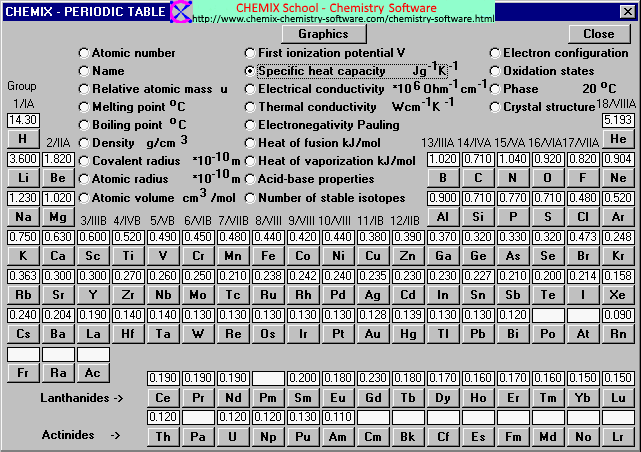
Table 1 from Molar Heat Capacity (Cv) for Saturated and Compressed Liquid and Vapor Nitrogen from 65 to 300 K at Pressures to 35 MPa | Semantic Scholar
Variation of Ideal Gas Heat Capacity Ratio with Temperature and Relative Density | Campbell Tip of the Month
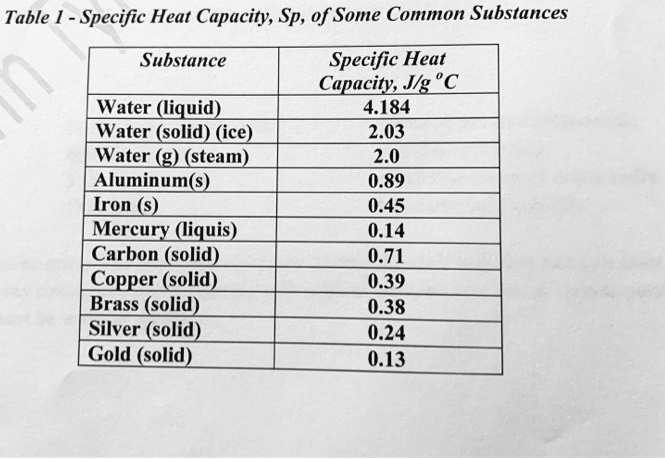
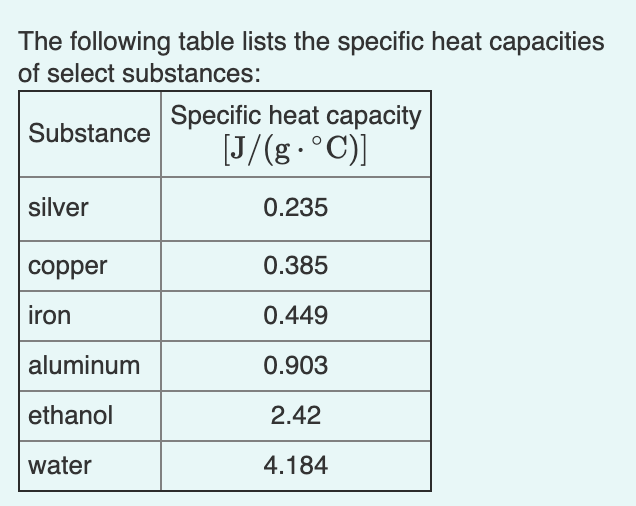
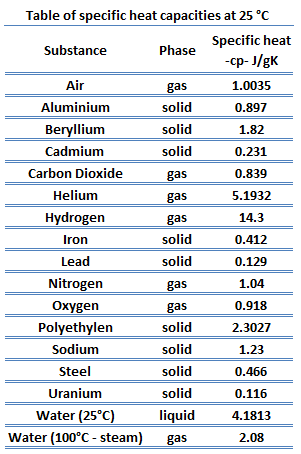
SOLVED: Table 1: Specific Heat Capacity, Sp, of Some Common Substances Substance Specific Heat Capacity, J/g 4.184 2.03 2.0 0.89 0.45 0.14 0.71 0.39 0.38 0.24 0.13 Water (liquid) Water (solid) (ice)

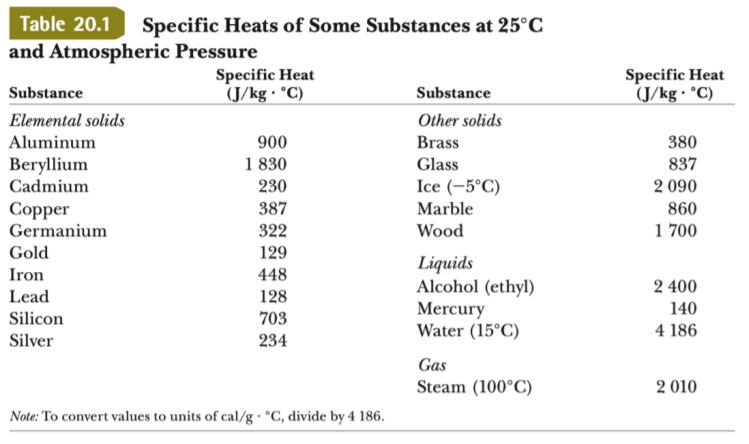
Table 2 from Heat capacities and thermodynamic properties of annite ( aluminous iron biotite ) | Semantic Scholar
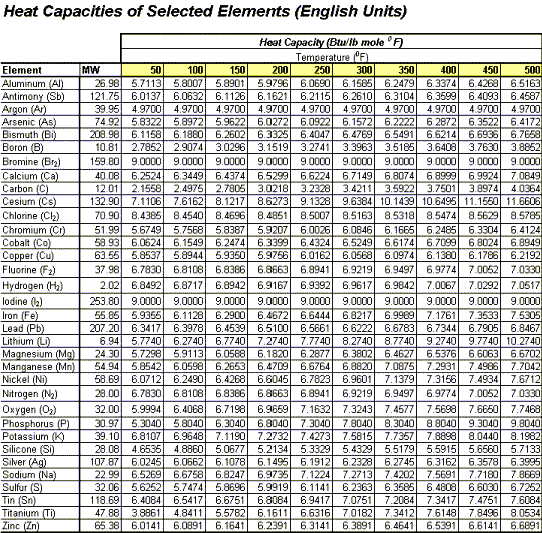
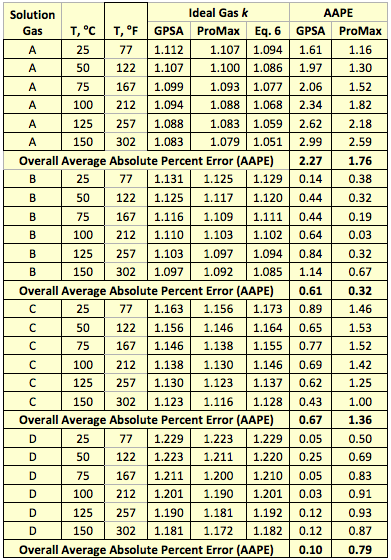
Estimating Heat Capacities for Solutions with Dissolved Solids - Calculations and Tips - Articles - Chemical Engineering - Frontpage - Cheresources.com
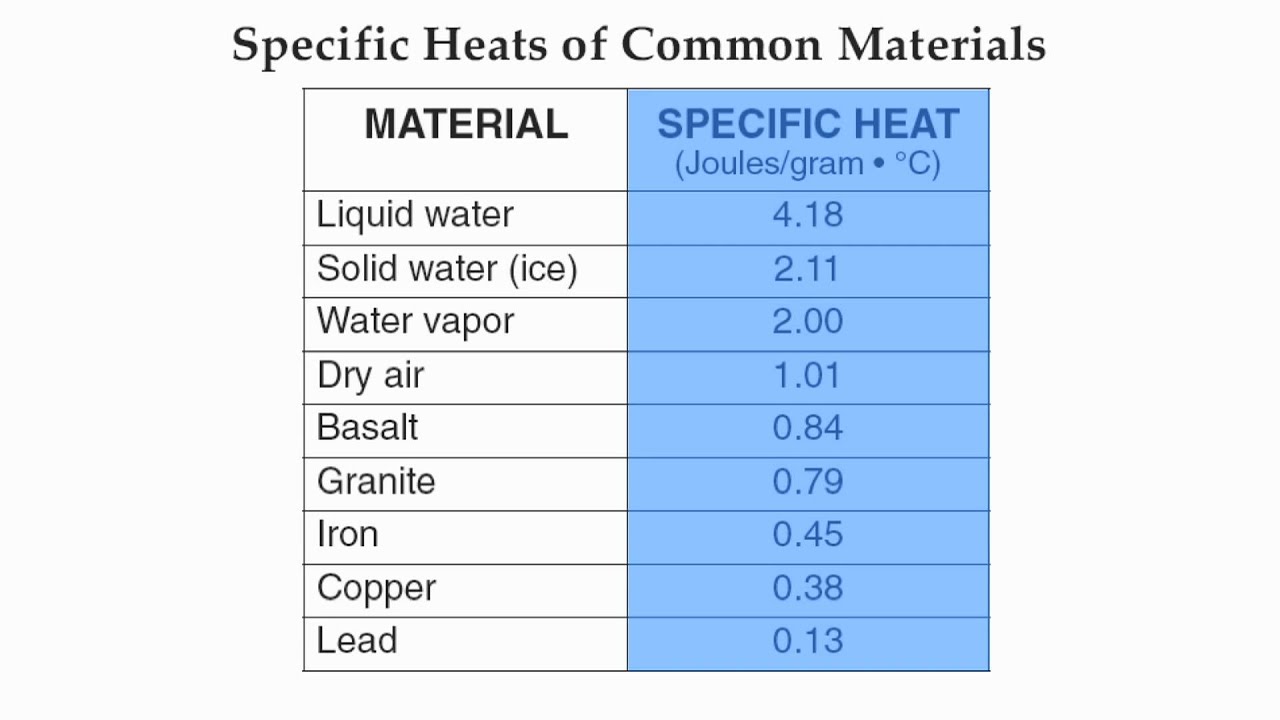
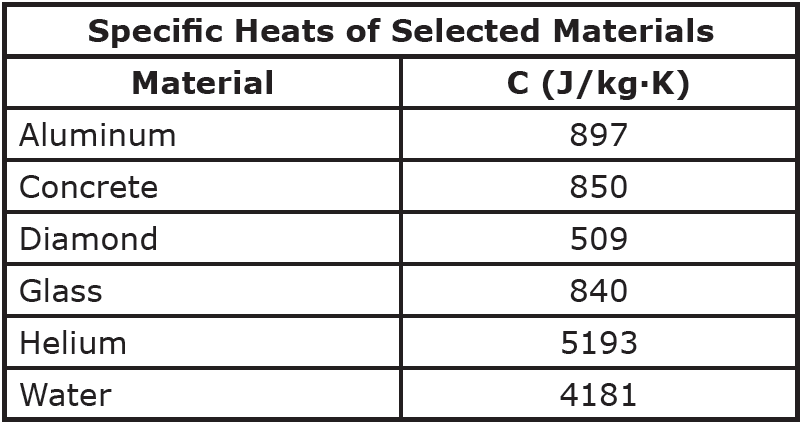
Reference Table Page 1-Specific Heat of Common Materials-Hommocks Earth Science Department - YouTube
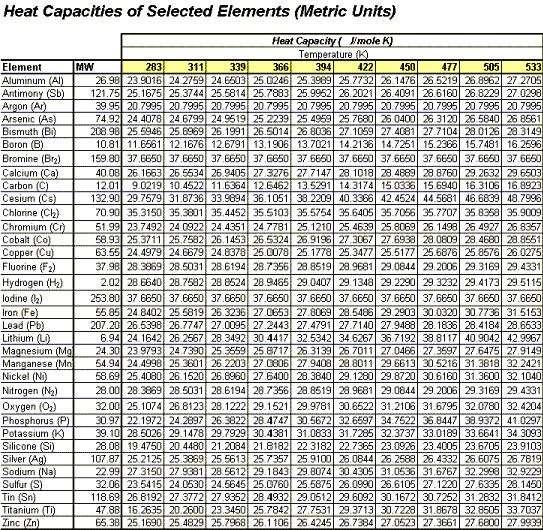
Estimating Heat Capacities for Solutions with Dissolved Solids - Calculations and Tips - Articles - Chemical Engineering - Frontpage - Cheresources.com
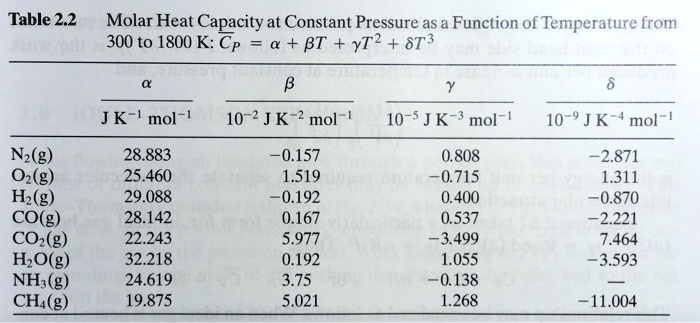
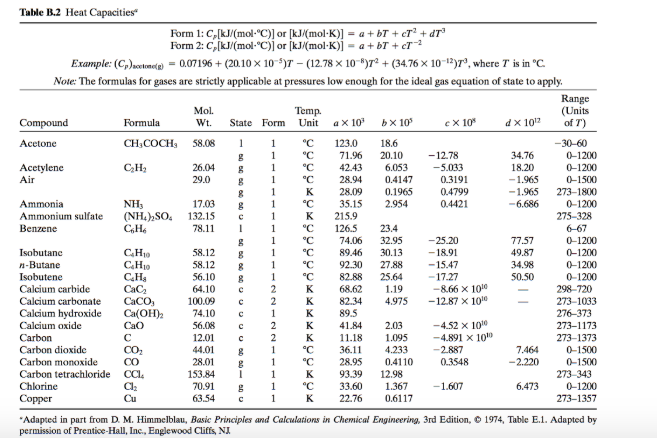
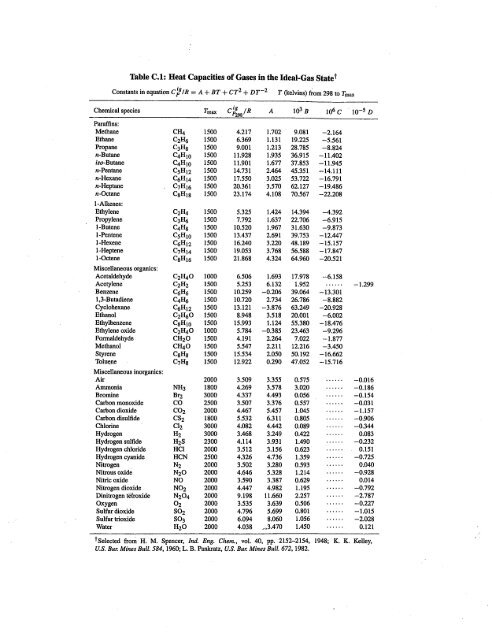
SOLVED: Table 2.2 Molar Heat Capacity at Constant Pressure as a Function of Temperature from 300 to 1800 K: Cp = a + BT + YT^2 + ST^3 JK mol^-1 10^-2 J K^-
Tables of Various Mach Number Functions for Specific-Heat Ratios From 1.28 to 1.38 - Page 19 of 77 - UNT Digital Library

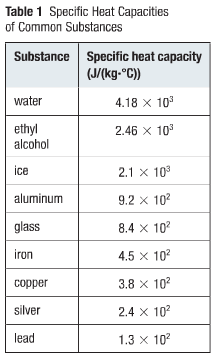
Table of Specific Heat Capacities: List of Thermal Conductivities | PDF | Molar Concentration | Temperature